The carbonaceous anode material has a small volume change during charge and discharge, and has good cycle stability energy, and the carbonaceous anode material itself is a mixed conductor of ions and electrons; in addition, silicon and carbon have similar chemical properties, and the two can be tightly combined. Therefore, carbon is often used as the preferred substrate for compounding with silicon.
With the rapid development of the times, the energy density of lithium-ion batteries is increasing at a rate of 7% to 10% per year. In 2016, China released the hard energy index of power battery. According to the “Technology Roadmap for Energy Saving and New Energy Vehiclesâ€, the energy density target of pure electric vehicle power battery in 2020 is 350W·h/kg.
In order to meet the needs of the new generation of energy, the development of new lithium battery negative technology is extremely urgent.
Silicon can be alloyed with lithium at room temperature to form a Li15Si4 phase with a theoretical specific capacity of up to 3572 mA·h/g, much higher than the theoretical specific capacity of commercial graphite (372 mA·h/g), and abundant in crust elements (26.4%). , the second place), low cost, environmentally friendly, so silicon anode materials have been the focus of researchers, is one of the most potential next-generation lithium-ion battery anode materials.
However, there is a serious volume expansion (~300%) in the charging and discharging process of silicon. The large volume effect and low conductivity limit the commercial application of silicon negative electrode technology. In order to overcome these shortcomings, researchers have made a lot of attempts to use composite technology to compensate for material expansion by using a "buffer skeleton."
The carbonaceous anode material has a small volume change during charge and discharge, and has good cycle stability energy, and the carbonaceous anode material itself is a mixed conductor of ions and electrons; in addition, silicon and carbon have similar chemical properties, and the two can be tightly combined. Therefore, carbon is often used as the preferred substrate for compounding with silicon.
In the Si/C composite system, Si particles act as active materials to provide lithium storage capacity; C can buffer the volume change of silicon negative electrode during charge and discharge, improve the conductivity of Si material, and avoid the charge of Si particles. Agglomeration occurs in the discharge cycle. Therefore, Si/C composites combine the advantages of both, exhibiting high specific capacity and long cycle life, and are expected to replace graphite as a new generation of lithium ion battery anode materials.
Starting from the structure of the silicon-carbon composite material, the currently studied silicon-carbon composite material can be divided into a cladding structure and an embedded structure. Among them, the cladding structure is coated with a carbon layer on the surface of the active material silicon to alleviate the volume effect of the silicon and enhance its conductivity. According to the coating structure and the morphology of the silicon particles, the coating structure can be classified into a core-shell type, an egg yolk-shell type, and a porous type.
Core-shell type
The core-shell silicon/carbon composite material is a silicon particle as a core and uniformly coated with a carbon layer on the outer surface of the core. The presence of the carbon layer is not only conducive to increasing the conductivity of silicon, buffering the partial volume effect of silicon in the process of deintercalating lithium, but also minimizing the direct contact between the silicon surface and the electrolyte, thereby alleviating the decomposition of the electrolyte and making the entire electrode Cycle performance is improved.
Zhang et al. used emulsion polymerization to coat polyacrylonitrile (PAN) on the surface of silicon nanoparticles and heat-treated at 800 °C to obtain a silicon-carbon core-shell composite (Si@C). The amorphous carbon layer inhibits the agglomeration of silicon particles during charge and discharge, and the capacity of Si@C is maintained at about 50% of the initial capacity after 20 cycles. In contrast, silicon nanoparticles have a significant capacity decay after 20 cycles.
Hwa et al. used polyvinyl alcohol (PVA) as a carbon source to carbon-coated silicon nanoparticles by high-temperature pyrolysis in an inert atmosphere to obtain a silicon-carbon composite with a carbon shell thickness of 5-10 nm. The use of silicon nanoparticles can reduce the absolute volume effect of silicon and weaken the internal stress of the material. The carbon coating further buffers the expansion of the silicon core. The composite material can still reach a capacity of 1800 mA·h after circulating 50 times at a current of 100 mA/g. /g, exhibiting excellent cycle stability, while pure nano-Si and carbon-coated micro-silicon (4 μm) capacity is attenuated to less than 200 mA·h/g.
Xu et al. obtained a core-shell type silicon-carbon composite material by high temperature thermal depolymerization of polyvinylidene fluoride (PVDF), the carbon layer thickness of which is 20~30nm; the silicon carbon composite electrode has a voltage range of 0.02~1.5V, 50mA/g current The first reversible specific capacity under the conditions was 1328.8 mA·h/g, and the capacity was maintained at 1290 mA·h/g after 30 cycles, and the capacity retention rate was 97%. In the core-shell silicon/carbon composites, the choice of different pyrolysis carbon source materials has different effects on the interface of silicon-carbon intercalation matrix in the composite system.
Liu et al. Comparatively analyzed silicon-based core-shell anode materials using polyethylene oxide (PEO), polyvinyl chloride (PVC), polyethylene (PE), chlorinated polyethylene (CPE) and PVDF as pyrolytic carbon sources. It is found that due to the etching effect of fluorine-containing materials on silicon, part of F can be embedded in the Si-Si bond, which effectively strengthens the interface compatibility between pyrolytic carbon and silicon core, and the corresponding Si-PVDF-based active material also exhibits More excellent cycle stability.
Therefore, when the carbon source organic precursor contains F or Cl element, it is advantageous to obtain a more stable silicon-carbon interface, and the electrochemical performance of the material is more excellent.
In summary, by coating the silicon material with carbon coating, the core-shell structure is built to help improve the cycle stability of the material. However, when the pyrolytic carbon in the silicon-carbon core-shell structure is coated on the surface of the silicon particles without voids, the volume effect of the silicon lithiation process is too large, which causes the entire core-shell particle to swell and even cause the surface carbon layer to occur. The rupture, the composite structure collapses, and the cycle stability decreases rapidly. In order to solve this problem, the researchers started from the aspect of strengthening the mechanical properties of the shell and designed a double-shell structure.
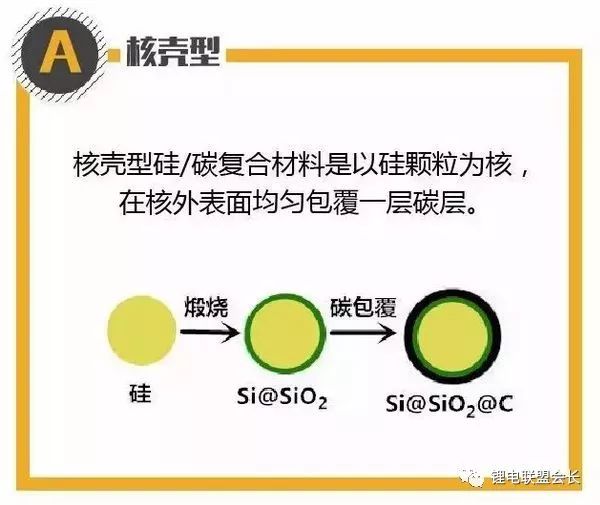
Tao et al. prepared a composite material with a double-shell structure (Si@SiO2@C) by coating SiO2 and pyrolytic carbon on the surface of silicon nanoparticles, as shown in Figure A. Compared with the single-shell Si@C, Si@SiO2@C has a higher capacity retention rate and still has a reversible capacity of 785 mA·h/g after 100 cycles in the voltage range of 0.01 to 5V.
The study shows that the intermediate layer SiO2 acts as a buffer phase, which can further reduce the expansion stress generated by the cyclic process. At the same time, the SiO2 layer can also react irreversibly with the diffused Li+ to form Si and Li4SiO4 alloys, further ensuring the reversible capacity of the material.
Comcn Electronics Limited , https://www.comencnspeaker.com